Round 3, Polymer 3: Iron to the Fore
Clifford E Carnicom
Mar 11 2024
A third polymer form has been isolated from the Cross Domain Bacteria (CDB) (nomenclature, 2014) culture processes. Please see recent research papers (1,2) to be aware of the precedent for this work. This compound is assessed as an organic iron complex (organometallic) with a polymeric structure. Protein structures are known to be developing in conjunction with the polymer layer, and as with prior polymers isolated, the material is regarded as unusual and distinct.
Third polymer form isolated from the CDB culture.
Organic, iron, and polymer characteristics dominate the composition.
Magnification 1x.
Biomedical applications (in this case, a.k.a. synthetic biology applications) are essentially guaranteed from the known origin and nature of this compound. Significant iron disruption mechanisms have been identified in the course of CI research, and this compound adds to that list. There are, as well, other iron compounds identified in recent work that can be presumed to trigger immune responses and significantly degrade blood oxygen carrying capacity.
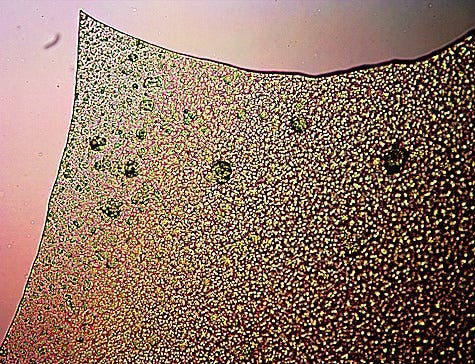
Assessed organometallic (iron) polymer from CDB culture.
Material has a film/gelatin like appearance, and separates into sheet form
on the slide with distinctive sharp boundaries.
Magnification 3200x.
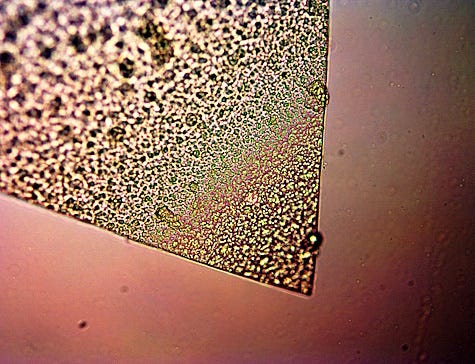
Assessed organometallic (iron) polymer from CDB culture.
Acute angle and sharp distinct boundaries are
common in the separation process.
Reasonably uniform interior structure.
Magnification 3200x.
______________________________________
End of Short Version
The methods of analysis include:
1. Measured or observed properties of a particular and specific material that are produced solely by the CDB in culture form.
2. Ultraviolet Spectroscopy (UV)
3. Near Infrared Spectroscopy (NIR)
4. Qualitative Chemistry
5. Conductivity
6. pH
7. Thermodynamics
8. Solubility
9. Spectroscopy and chemical reference sources.
10. Microscopic analysis
11. Reasonably sophisticated chemometric analysis that attempts to synthesize the chemical nature of the material in a combined sense, dependent upon measured properties.
The measured or observed properties of the substance are:
1. Insoluble in H2O.
2. Insoluble in H2SO4 approx 1M.
3. Significant reaction in NaOH-KOH. Remains insoluble but immediately oxidizes to a rich brown color. Essentially certain to be an iron complex of some sort.
4. Protein or polymer suspected.
5. Originates from genetically engineered bacteria that is known to utilize ferrous iron and oxidize it to ferric form.
6. Material forms on surface solution and is presumed to be less dense than water.
7. Material has a gelatin appearance.
8. Under microscope at 3200x, material breaks into thin layer of material with smooth boundaries and acute angles of separation. Might be similar to expected behavior of gelatin or acetate nature.
9. Fully soluble and transparent in strong H2SO4, estimated 5M.
10. Well defined UV absorbance at 302 nanometers (nm) and at 226 nm.
11. A local minimum of UV absorbance occurs at approximately 273 nm.
12. Near Infrared absorbance at less than or equal to 900 nm.
13. Near Infrared absorbance at approximately 1412 nm.
14. Near Infrared absorbance at approximately 1526 nm.
15. Near Infrared absorbance at approximately 1665 and 1700 nm.
16. There is a local minimum of near infrared absorbance at approximately 1211 nm.
17. Material browns at 315 deg C
18. Material charred at 350 deg C
19. The blackened material has flakes that are reflecting light, as if they are crystallized , at approximately 460 deg C.
20. The combustion point, if it exists, is greater than 570 deg C.
21. No smoke is visible during the heating process.
22. The gelatin appearing surface layer formed after addition of ferrous iron to a preexisting culture state.
23. The pH of the culture which produces this material is 3.7.
24. The electrical conductivity of the culture which produces this material is approximately 8.6 millisiemens (mS).
25. The material dries rapidly under mild heat and easily reduces to a powder form.
26. Amide and/or Amine groups are probable to exist due to near infrared absorption measurements.
A future paper will discuss the synthesis of impact from the three CDB polymer forms that are now isolated.
Clifford E Carnicom
Mar 11 2024
born Clifford Bruce Stewart, Jan 19, 1953





Questions : If iron is being used by these structures should we (the people) be supplementing with iron due to cellular depletion by the CDB? Or is this a bad idea and will only "feed" the CDB?
Thank you so much for your work.
So I’ve noticed in my labs and the labs of my husband and friend, we all have high iron and iron saturation and low TIBC and UIBC, I assume this is in response to iron depletion and it’s a paradoxical response. What’s the best way to counter this? Should we be consuming higher iron foods?